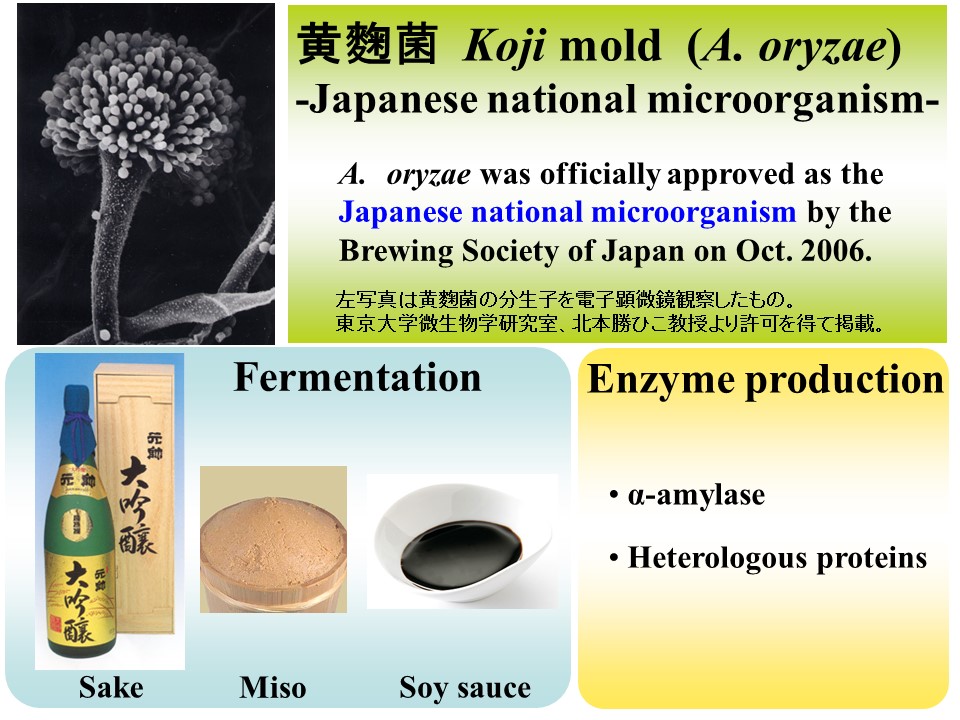
Why do we use A. oryzae?
Aspergillus oryzae is a very useful microorganism that has been used in Japanese fermentation industry to produce sake, miso and shoyu. Therefore, the fungus is recognized as GRAS(Generally Regarded As Safe) and has been officially approved as the Japanese national microorganism by the Brewing Society of Japan on October 2006. A. oryzae can secrete a large amount of proteins, such as alfa-amylase. Using this feature, valuable proteins that are expressed in A. oryzae have been successfully produced.
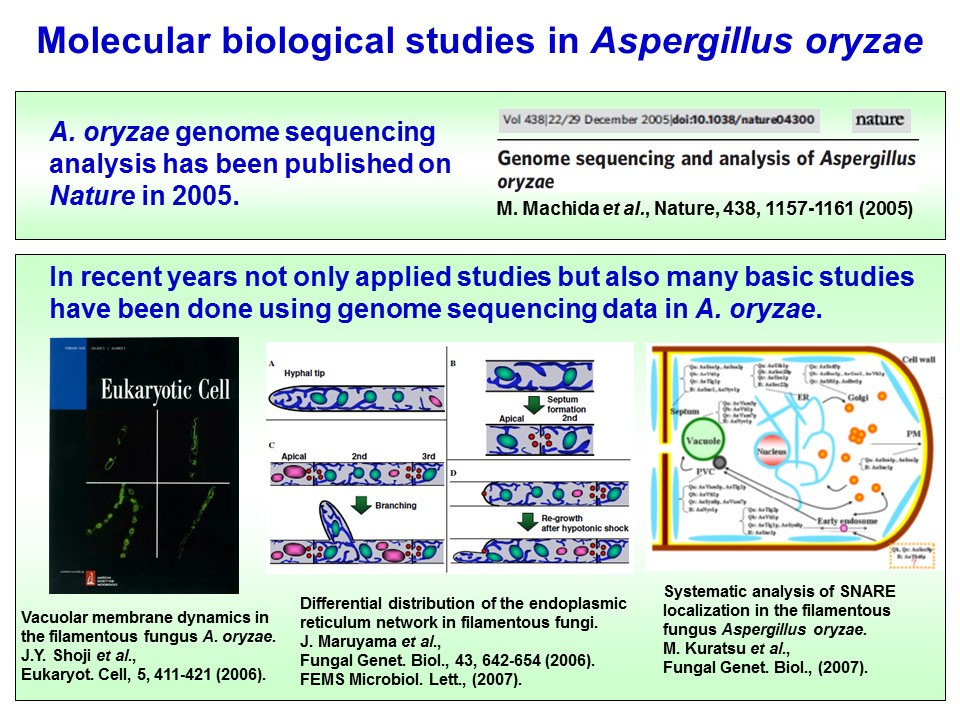
A. oryzae as a model organism
As described above, A. oryzae was known since old times, but sexual generation has not yet been confirmed, which makes this fungus arduous to handle by traditional genetic research methods. Furthermore, A. oryzae is a multi-cellular organism with several nuclei, which also makes this microbe hard to use. Thus, A. oryzae had been analyzed only in applied biology, but not in fundamental biology. However, in 2005, A. oryzae genome sequencing analysis has been completed and published in Nature, conducted by the Japanese consortium of A. oryzae genome analysis. Using this very useful information, reverse genetics have become to be easily done (Machida et al., 2005). Moreover, molecular biological techniques have been established, such as the Multi-Site Gateway system that allows generation of expression plasmids much easier and quicker (Mabashi et al., 2006). Together with Neurospora crassa and Aspergillus nidulans whose genomes have also been already analyzed, A. oryzae has been recognized as a model organism of filamentous fungi with respect to both in applied and basic sciences. In fact, by cellular biological approaches using GFP (Green Fluorescence Protein), organelles and thier marker proteins have been well analyzed (Maruyama et al., 2006; Shoji et al., 2006; Kuratsu et al., 2007).
Valuable heterologous protein production in A. oryzae
Is it indeed easy to produce plenty of valuable heterologous proteins by A. oryzae cells? Unfortunately, the answer is "No" so far. Generally, to introduce genes encoding valuable heterologous proteins into A. oryzae renders to obtain much less amount of proteins than native ones. It is likely because heterologous proteins are not efficiently translated and/or secreted and can be degraded by extracellular proteases in the culture supernatant. In fact, by removing such potential hindrances, there are many reports in which valuable proteins could be produced more (Nemoto et al., 2009; Yoon et al., 2010; Ohno et al., 2011; Yoon et al., 2011).
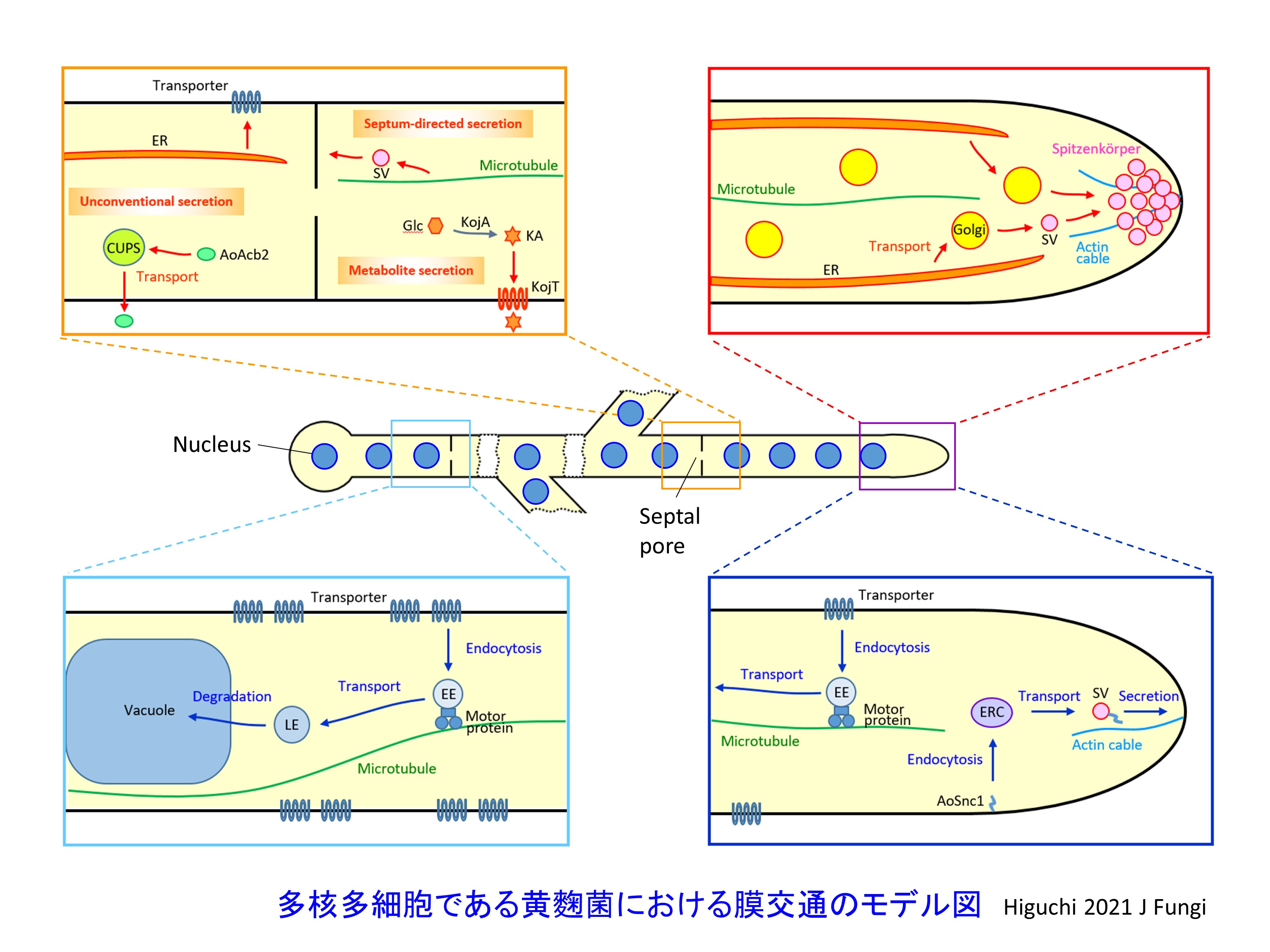
How to produce valuable heterologous proteins in A. oryzae? ~Approach by cell biology~
Then hereafter, how valuable heterologous proteins can be produced in A. oryzae? We focus on protein secretion pathway. After synthesized by ribosomes, secretory proteins are transported through endoplasmic reticulum (ER) and Golgi aparatus and secreted from the plasma membrane to outside the cells. In these steps, secretory proteins are transported inside vesicles coated with lipid bilayer, the process of which is called vesicular trafficking. Intracellular vesicular trafficking pathway includes not only secretory pathway but also endocytic pathway, by which molecules are incorporated from the plasma membrane into inside cells. In addition, intracellular vesicular trafficking pathway is important not only for trafficking molecules but also for maintaining the balance of lipid bilayer inside cells. Therefore, by analysing endocytic pathway as well as secretory pathway, we are trying to find out a new approach for valuable heterologous protein production.
Study of endocytic pathway in A. oryzae
In filamentous fungi, including A. oryzae, studies about endocytosis had not been done well (Read and Kalkman, 2003). Therefore, we first constructed an experimental method to visualise endocytosis using a GFP-fused plasma membrane protein (Higuchi et al., 2006). Then, we demonstrated that endocytosis is closely related to secretion in A. oryzae elucidated by the physiological study of endocytosis (Higuchi et al., 2009a; Higuchi et al., 2009b). From now on as described above, it is crucial how to apply the basic knowledge about endocytosis to valuable heterologous protein production. Currently, however, still little is known about endocytosis in A. oryzae. On the other hand, since detailed molecular mechanisms have not been clarified in A. oryzae, we consider that fundamental studies about endocytosis are very intriguing (Higuchi et al., 2011; Matsuo et al., 2013). Recently in other filamenotus fungus, the motility of early endosomes, organelles of endocytic pathway, is important for distribution of polysomes, complexes for protein translation (Higuchi et al., 2014; summarised in Higuchi and Steinberg, 2015). In this respect, by tuning endocytic pathway, we are also aiming to develop an A. oryzae strain that can produce more and more valuable heterologous proteins.
Publications
*corresponding author
18. Y. Higuchi*, K. Takegawa.
Single-molecule FISH reveals subcellular localization of α-amylase and actin mRNAs in the filamentous fungus Aspergillus oryzae.
Frontiers in Microbiology, 11, 578862 (2020)
17. Y. Morita, F. Kikumatsu, Y. Higuchi*, Y. Katakura, K. Takegawa.
Characterization and functional analysis of ERAD-related AAA+ ATPase Cdc48 in Aspergillus oryzae.
Fungal Biology, 124, 801-813 (2020)
16. Q. Li, Y. Higuchi*, K. Tanabe, Y. Katakura, K. Takegawa.
Secretory production of N-glycan-deleted glycoprotein in Aspergillus oryzae.
Journal of Bioscience and Bioengineering, 129, 573-580 (2020)
15. Y. Togo, Y. Higuchi*, Y. Katakura, K. Takegawa.
Early endosome motility mediates α-amylase production and cell differentiation in Aspergillus oryzae.
Scientific Reports, 7, 15757 (2017)
14. H.S. Kwon, K. Kawaguchi, T. Kikuma, K. Takegawa, K. Kitamoto*, Y. Higuchi*.
Analysis of an acyl-CoA binding protein in Aspergillus oryzae that undergoes unconventional secretion.
Biochemical and Biophysical Research Communications, 493, 481-486 (2017)
13. K. Kawaguchi, T. Kikuma, Y. Higuchi*, K. Takegawa, K. Kitamoto*.
Subcellular localization of acyl-CoA binding protein in Aspergillus oryzae is regulated by autophagy machinery.
Biochemical and Biophysical Research Communications, 480, 8-12 (2016)
12. M. Schuster, M. Martin-Urdiroz, Y. Higuchi, C. Hacker, S. Kilaru, S. Gurr, G. Steinberg.
Co-delivery of cell wall-forming enzymes in the same vesicle for coordinated fungal cell wall formation.
Nature Microbiology, 1:16149 (2016)
11. Y. Higuchi.
Initial fungal effector production is mediated by early endosome motility.
Communicative and Integrative Biology, 8, e1025187 (2015)
10. Y. Higuchi*, G. Steinberg*.
Early endosomes motility in filamentous fungi: How and why they move.
Fungal Biology Reviews, 29, 1-6 (2015)
9. E. Bielska#, Y. Higuchi#, M. Schuster#, N. Steinberg, S. Kilaru, N.J. Talbot, G. Steinberg.
Long-distance endosome traffiking drives fungal effector production during plant infection.
Nature Communications, 5, 5097 (2014) #These authors contributed equally.
8. Y. Higuchi, P. Ashwin, Y. Roger, G. Steinberg.
Early endosome motility spatially organizes polysome distribution.
Journal of Cell Biology, 204, 343-357 (2014)
7. K. Matsuo, Y. Higuchi, T. Kikuma, M. Arioka, K. Kitamoto.
Functional analysis of Abp1p-interacting proteins involved in endocytosis of the MCC component in Aspergillus oryzae.
Fungal Genetics and Biology, 56, 125-134 (2013)
6. Y. Higuchi, M. Arioka, K. Kitamoto.
Functional analysis of the putative AAA ATPase AipA localizing at the endocytic sites in the filamentous fungus Aspergillus oryzae.
FEMS Microbiology Letters, 320, 63-71 (2011)
5. K. Takaya, Y. Higuchi, K. Kitamoto, M. Arioka.
A cytosolic phospholipase A2-like protein in the filamentous fungus Aspergillus oryzae localizes to the intramembrane space of the mitochondria.
FEMS Microbiology Letters, 301, 201-209 (2009)
4. Y. Higuchi, M. Arioka, K. Kitamoto.
Endocytic recycling at the tip region in the filamentous fungus Aspergillus oryzae.
Communicative and Integrative Biology, 2, 327-328 (2009b)
3. Y. Higuchi, J.Y. Shoji, M. Arioka, K. Kitamoto.
Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae.
Eukaryotic Cell, 8, 37-46 (2009a)
2. R. Hatakeyama, T. Nakahama, Y. Higuchi, K. Kitamoto.
Light represses conidiation in koji mold Aspergillus oryzae.
Bioscience, Biotechnology, and Biochemistry, 71, 1844-1849 (2007)
1. Y. Higuchi, T. Nakahama, J.Y. Shoji, M. Arioka, K. Kitamoto.
Visualization of the endocytic pathway in the filamentous fungus Aspergillus oryzae using an EGFP-fused plasma membrane protein.
Biochemical and Biophysical Research Communications, 340, 784-791 (2006)
総説・著書
樋口裕次郎、北本勝ひこ
麹菌のタンパク質分泌経路とエンドサイトーシス
発酵・醸造食品の最新技術と機能性II、シーエムシー出版、78-85 (2011)
正路淳也、樋口裕次郎、丸山潤一、北本勝ひこ
糸状菌オルガネラの形態とその極性依存的な配置
蛋白質核酸酵素、53、753-759 (2008)
正路淳也、樋口裕次郎、丸山潤一
麹菌を用いた糸状菌細胞生物学の進展
日本生物工学会誌、84、364-366 (2006)
International conference oral presentations
Y. Higuchi, P. Ashwin, G. Steinberg.
The cellular role of early endosome motility in Ustilago maydis.
27th Fungal Genetics Conference, Asilomar Conference Grounds, USA (March 2013)
Y. Higuchi, J.Y. Shoji, M. Arioka, K. Kitamoto.
Analysis of the endosomal compartments in the filamentous fungus Aspergillus oryzae.
3rd Physiology of Yeasts and Filamentous Fungi, Helsinki, Finland (June 2007)
Y. Higuchi, J.Y. Shoji, M. Arioka, K. Kitamoto.
Visualization of the endocytic pathway and endosomal structures in the filamentous fungus Aspergillus oryzae.
8th International Mycological Congress, Cairns, Australia (August 2006)
Contact
Yujiro Higuchi: y.higuchiアットagr.kyushu-u.ac.jp (change アット to @)